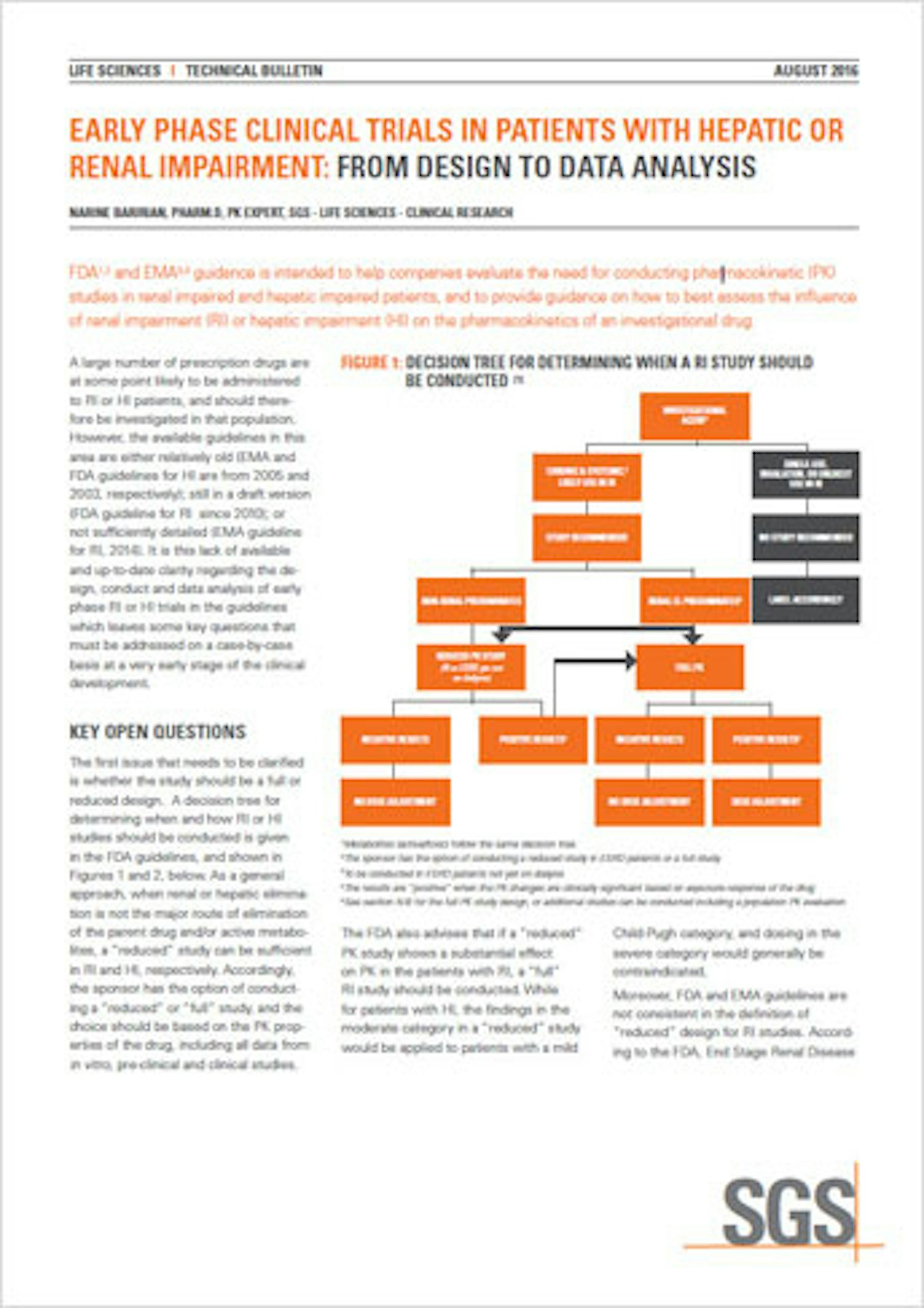
FDA and EMA guidances are intended to help companies evaluate the need for conducting pharmacokinetic studies in renal and hepatic impaired patients and to provide guidance on how to best assess the influence of renal impairment or hepatic impairment on the pharmacokinetics of an investigational drug. However, while many procedural recommendations exist, some key questions still need to be investigated case-by-case at the early stage of the project development.
This white paper will address key questions that arise in this type of clinical trial, from design to data analysis.
This white paper will address key questions that arise in this type of clinical trial, from design to data analysis.