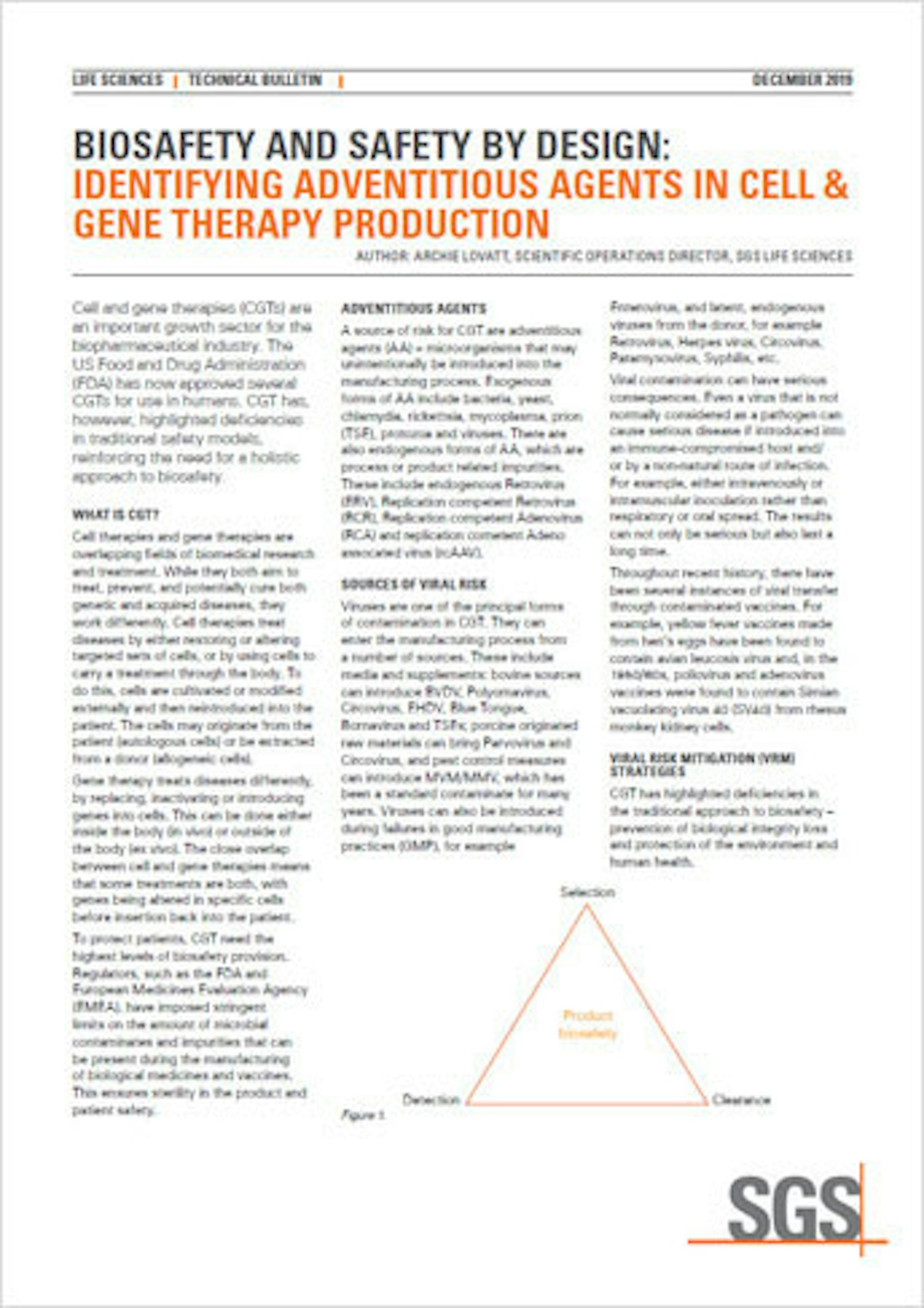
In this document we look at cell and gene therapies (CGTs), an important growth sector for the biopharmaceutical industry. The US Food and Drug Administration (FDA) has now approved several CGTs for use in humans. CGT has, however, highlighted deficiencies in traditional safety models, reinforcing the need for a holistic approach to biosafety.