Introduction
As a medical device Notified Body (SGS Belgium NV – Notified Body 1639), we are accredited to certify all types of medical devices, including those without an intended medical purpose. Once certified with us, you will be entitled to use the CE 1639 mark on the devices and their labeling before placing the devices on the European Union market.
All devices certified by SGS NB 1639 require you to get an EU Quality Management System (QMS) certificate. Before using the CE 1639 mark, Class III and implantable Class IIb1 must also have an EU Technical Documentation Assessment certificate.
A list of standard fees for conformity assessment activities under MDR (2017/745) is available from our EU Medical Devices Regulations Information Center.
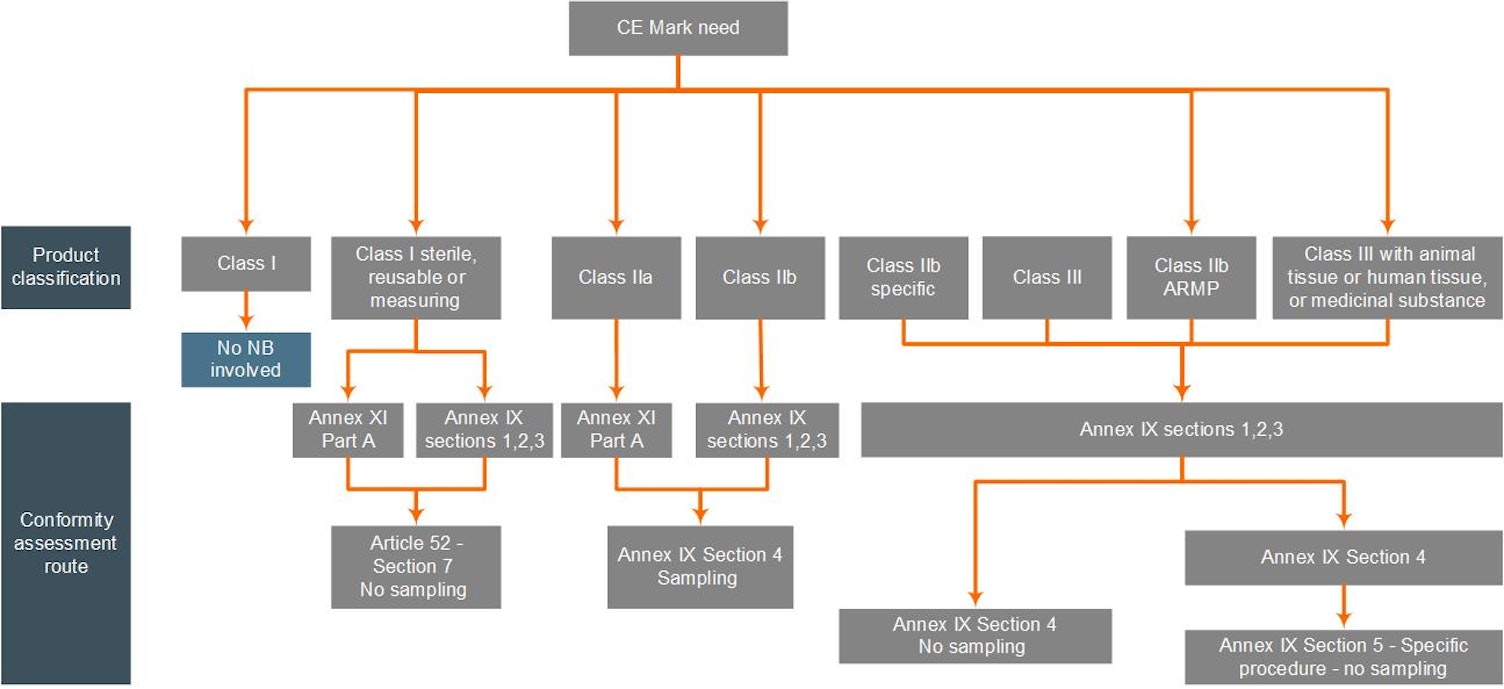
The first step will be for you to determine your product(s) classification according to the rules defined in Annex VIII of the MDR. Subsequently, you must decide which type of conformity assessment path you wish to apply, which is either:
- Conformity assessment based on the QMS and Technical Documentation Assessment as per Annex IX of the MDR
- Conformity assessment based on Product Conformity Verification (Product Quality Assurance) as per Annex XI Part A of the MDR
For devices that are “medicinal” and governed by Directive 2001/83/EC, according to Article 8 of the MDR, we can propose an assessment, according to MDR Article 117, to provide a Notified Body opinion on compliance with GSPR for the medical device part of a drug-device combination. We can also provide QMS certificates to distributors or importers carrying out any activities mentioned in points (a) and (b) of MDR Article 16(2), subject to an application and audit procedure. The sections applicable to these types of assessments will be indicated in this document.
The diagrams below present the type of conformity assessment per device class and guide you through the appropriate certification process that we may offer. For this diagram:
- “Class I Reusable” is an abbreviation of “Class I reusable surgical instruments”
- "Class IIb Specific” are implantable Class IIb devices, except for sutures, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips and connectors, which are subject to sampling
On this page
- Important Information
- Lodging Your Application
- Pre-Application Review
- Contract Proposal
- Application Review
- Stage 2 Audit
- Technical Documentation Assessment
- Nonconformance and Corrective Actions Request
- Certification Review
- Unannounced Audit After CE Certification
- Recertification
- Notification of Changes
- Vigilance
- Summary of Safety and Clinical Performance (SSCP)
- Periodic Safety Update Report (PSUR)
- Voluntary Change of Notified Body
- Additional SGS Medical Device Certification Services
- Useful References
- Annex 1: Changes That Must Be Notified to SGS Before Implementation
- Annex 2: Corrective Action Request (CAR)
- Footer
Loading component...
Loading component...
Loading component...
Loading component...
Loading component...
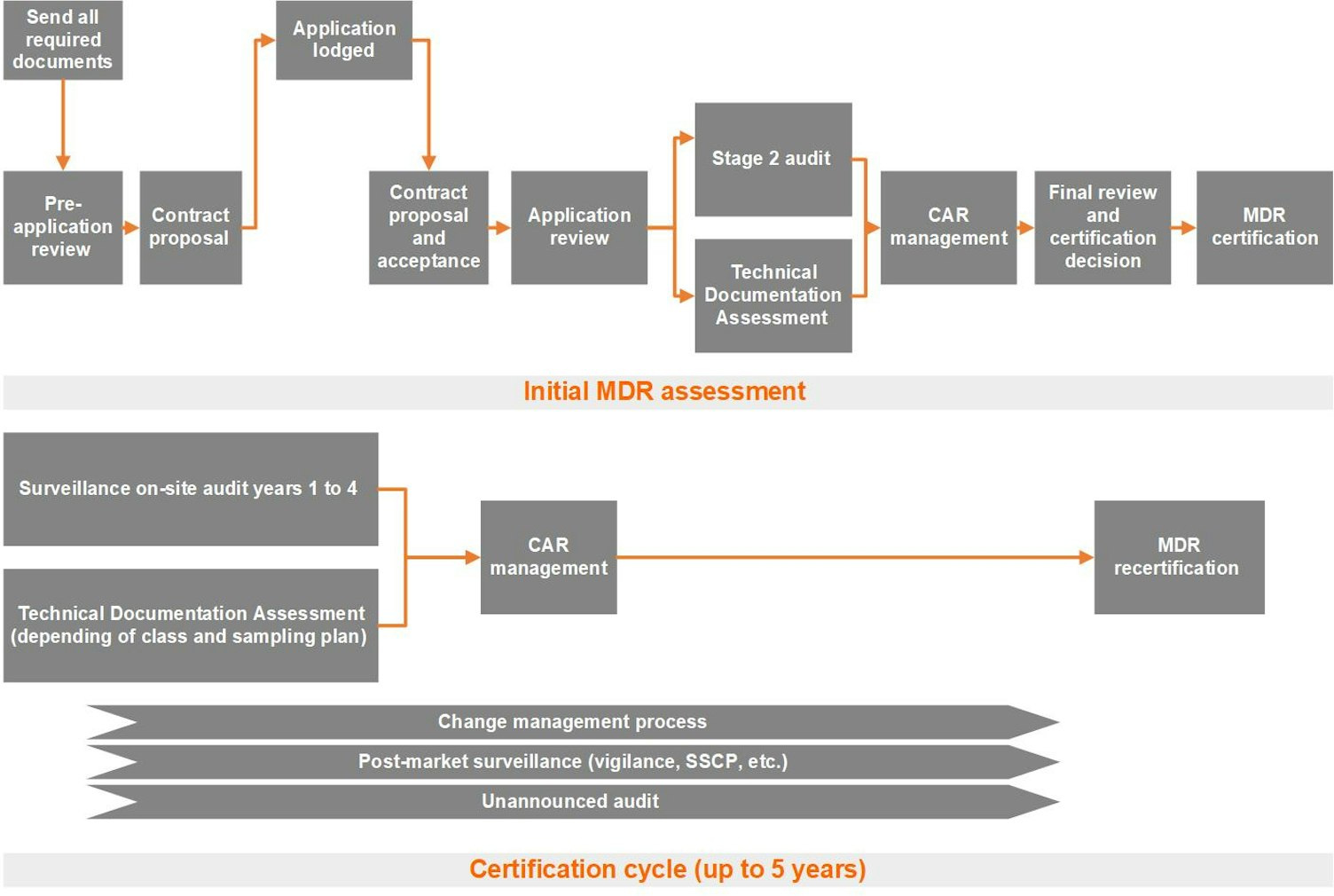
Stage 2 audit
This section does not apply to assessments conducted according to MDR Article 117.
This step is usually conducted several weeks after the Stage 1 audit to ensure that you have sufficient time to implement the Stage 1 audit findings. We are led by you regarding the time between Stage 1 and Stage 2 activities, but four weeks minimum would be recommended, and both stages should be planned well in advance.
A Stage 2 audit is performed on-site or as a hybrid audit (partially on-site and partially remote) and determines compliance against your documented QMS under the MDR (EU) 2017/745. This audit will also confirm the status of relevant suppliers and subcontractors, your critical processes and the eligibility of your products for medical device certification.
All assessment conclusions are based on a sampling of audit evidence to demonstrate effective implementation of the management system, control over the processes and progress made toward achieving your stated quality objectives and compliance with the MDR (EU) 2017/745.
Following the audit, the audit team will make a recommendation, dependent on the findings and the submission of corrective action plans for any nonconformances (see section on Non-Conformance and Corrective Action Requests). The auditor will talk you through the findings that may comprise major and minor nonconformances. The auditor will also agree with you on the name, address and proposed scope details that will appear on your certificates.