The Saudi Arabian cosmetic market is a powerhouse of opportunity, driven by a young, discerning population and a rapidly growing beauty sector. However, the gateway to this lucrative market is guarded by an evolving, stringent regulatory framework administered by the Saudi Food and Drug Authority (SFDA). For exporters, recent updates to the conformity assessment process have added new layers of complexity to market entry.
The key to success lies in working with a trusted partner who goes beyond interpreting the rules and is officially recognized by the SFDA to support your compliance journey. As an SFDA-approved Conformity Assessment Body, we guide you through every stage of the approval process, transforming regulatory complexity into business opportunity.
Understanding Saudi cosmetic product regulations
The SFDA mandates that all products must be safe and comply with specific technical requirements before entering the Kingdom. The cornerstone of this process is the consignment certificate of conformity (CoC).
The CoC program safeguards both consumers and the national market by ensuring all imported cosmetics, personal care and perfumery products meet SFDA safety and quality standards.
We certify cosmetic consignments to ensure your products are evaluated against the latest SFDA standards, regulations and requirements.
Key program requirements:
- Mandatory certification: every shipment must be accompanied by a valid CoC
- Customs clearance: consignments without a CoC will be denied entry and will not clear Saudi Customs
- Standard compliance: shipments that fail to meet assessment requirements will be rejected at the destination
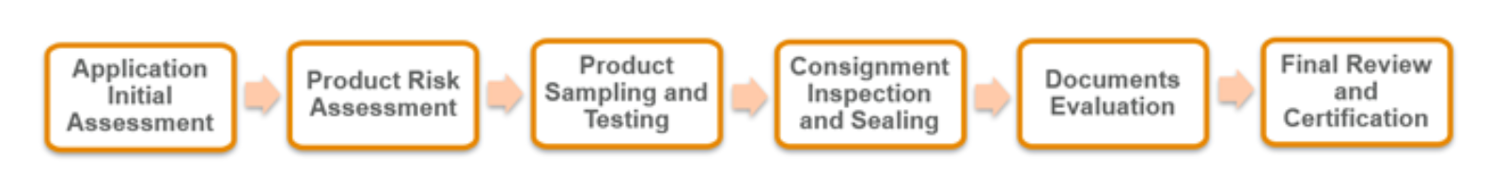
Key regulatory updates
A. Requirements under the new SFDA guidelines
Product requirements and standards
Products must comply with requirements in the following technical regulations and any additional circulars and notifications issued by the SFDA:
- Executive regulation for cosmetic products system
- SFDA CO/GSO 1943
- SFDA CO/GSO 2528
- ISO 22716 – Good Manufacturing Practices for Cosmetics
- Lists of banned and restricted cosmetic substances colorants, preservatives and UV filters
- Circulars published on the SFDA website
- Conditions for clearance of cosmetic products and raw materials used in their manufacture
Consignment inspection requirements
Inspection activities include:
- Onsite verification of the SFDA/GHAD registration number, ensuring the shipped product matches the approved version in the SFDA system
- Physical inspection of the product, packaging and labeling to confirm alignment with technical documentation
- Verification that the consignment complies with applicable regulations and clearance conditions
- Sample collection and testing, when required, under a verified chain of custody
- Inspection of transportation and containers to ensure safety, compliance, seal integrity and prevent tampering
B. Newly restricted substances
On August 14, 2025, the SFDA issued a circular adding three substances to its restricted ingredients list, setting new concentration limits. From January 1, 2026, importing or manufacturing non-compliant products will be prohibited. However, a grace period allows products already on the market to be sold until January 1, 2028, while compliance is achieved.
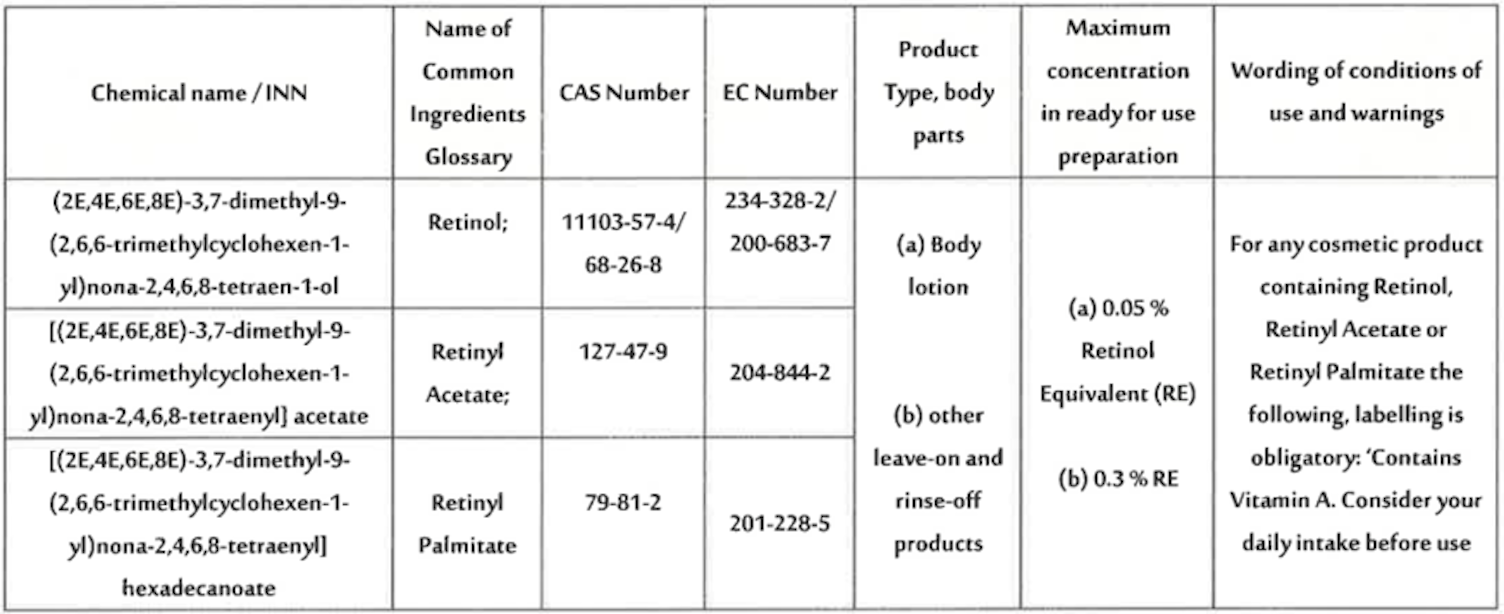
C. Newly prohibited substances
On September 7, 2025, the SFDA expanded its prohibited substances list to include 21 new ingredients, such as dibutyltin maleate, dibutyltin oxide, 4-nitrosomorpholine and 4-methylimidazole. This update aligns Saudi regulations with global safety standards and requires immediate reformulation and compliance reviews.
We proactively monitor regulatory changes and provide comprehensive ingredient screening of your formulations to identify any non-compliant materials. Our experts offer clear guidance on reformulation and documentation updates to maintain market continuity and ensure seamless adaptation without supply chain disruption.
SGS solutions: streamlining exports to Saudi Arabia
Expanding into Saudi Arabia’s cosmetics market demands precision, speed and compliance. As an SFDA-approved Conformity Assessment Body, we simplify the process, ensuring your products meet all regulatory requirements and reach consumers faster.
Key benefits:
- Proactive compliance – we act as your regulatory partner, monitoring SFDA updates and guiding you through any necessary product or documentation changes to keep your portfolio market-ready
- Accelerate market access – our deep understanding of SFDA expectations and efficient assessment process reduce approval delays, helping you protect timelines and revenue
- Trusted certification – a CoC issued by us is a mark of safety and quality, strengthening consumer and retailer confidence in a crowded marketplace
With SGS, you gain more than certification, you gain a partner committed to your long-term success. We help you navigate complex regulations with confidence so you can focus on what matters the most: growing your business in the Kingdom of Saudi Arabia.
Ready to simplify your market access? Contact our cosmetic regulatory experts today.
Learn more about Saudi Arabia – SFDA Certificate of Conformity.
This article can also be found in our PCA Newsletter (Q4/2025), which keeps you up to date with developments in technical barriers to trade and product conformity assessment.
Read more PCA articles (Q4/2025)
- UNBS Strengthens Controls on Certificates of Conformity and Updates Compulsory Standards
- Supporting Global Brands at Saudi Lifestyle Week 2025
- Key Updates on Kenya’s PVoC Program
- Tanzania Launches CoC Update and System Integration for Stronger PVoC Oversight
You can read more articles in our previous editions in the PCA Newsletter Library.
© SGS Société Générale de Surveillance SA.
400 Broadacres Drive,
Suite 200, 2nd Floor,
Bloomfield, New Jersey, NJ200,
United States


