Commission Regulation (EU) 2021/850 was published on May 28, 2021 to uniformly implement the prohibition and restriction of CMR substances within the internal market. Annex II, Annex III, Annex IV and Annex VI to Cosmetic Regulation (EC) 1223/2009 will therefore be updated.
In response to the carcinogenic, mutagenic, or toxic (CMR) classification of certain substances in Regulation (EU) 2020/217, Commission Regulation (EU) 2021/850 is published on May 28, 2021, to uniformly implement the prohibition and restriction of CMR substances within the internal market. Annex II, Annex III, Annex IV and Annex VI to Cosmetic Regulation (EC) 1223/2009 will be therefore updated.
Commission Regulation (EU) 2021/850 of May 26, 2021 amending and correcting Annex II and amending Annex III, IV, and VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products.
(A)
The following substances are classified as CMR in Delegated Regulation (EU) 2020/217. Without any request for use in cosmetic products by way of exception in Article 15(1) of (EC) 1223/2009, these substances are therefore added into the prohibition list in cosmetic regulation.
The prohibition will be applied from October 1, 2021.
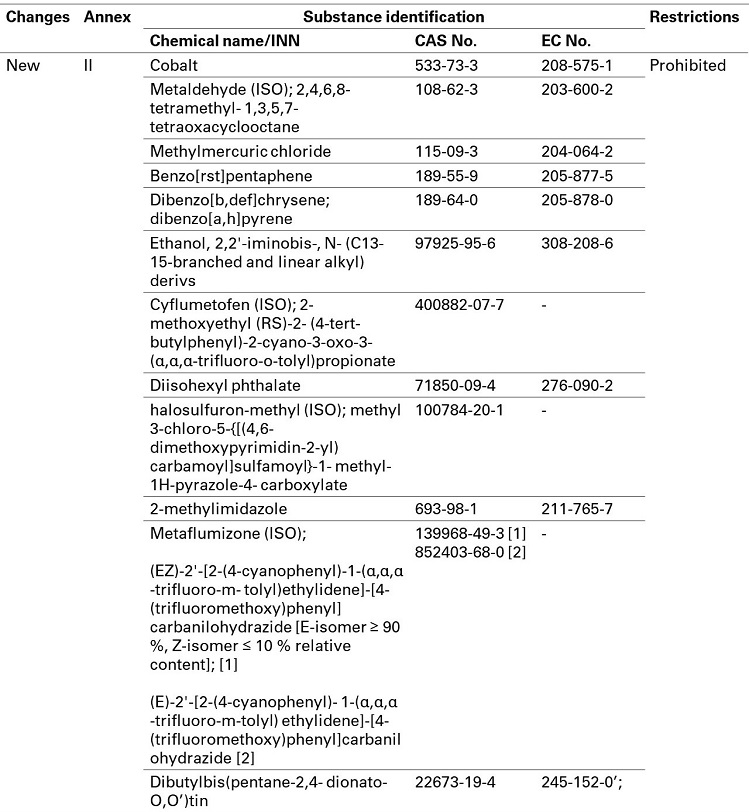
(B)
Titanium Dioxide (in powder form containing 1% or more of particles with an aerodynamic diameter of ≤10µm) is classified as Carcinogen Category 2 (inhalation) in Delegated Regulation (EU) 2020/217.
A request for its use in cosmetic product as an exception was submitted.
With the conclusion of SCCS/1617/20 opinion on Titanium dioxide (TiO2) published on October 6, 2020, the use of Titanium Dioxide (in powder form containing 1% or more of particles with aerodynamic diameter of ≤10µm) shall be allowed only in face products in loose powder form and in hair aerosol spray products. Its restriction is therefore added into Annex III to (EC) 1223/2009. The new restriction will be applied from October 1, 2021.
| Changes | New | |
| Annex | III | |
| No. | X | |
| Substance Identification | Chemical name/INN | Titanium dioxide in powder form containing 1 % or more of particles with aerodynamic diameter ≤ 10 μm |
| INCI name | Titanium Dioxide | |
| CAS no. | 13463-67-7/1317-70-0/1317-80-2 | |
| EC no. | 236-675-5/215-280-1/215-282-2 | |
| Restrictions | Product type, Body parts | (a)Face products in loose powder form (b)Hair aerosol spray products (c)Other products |
| Max. concentration in ready for use preparation | (a)25% (b)1.4% for general consumers and 1.1% for professional use | |
| Other | (a), (b) Only in the pigmentary form (c) Not to be used in applications that may lead to exposure of the end-user’s lungs by inhalation’ | |
| Wording of conditions of use and warnings | ||
The use of Titanium Dioxide in Annex VI as a UV filter should therefore be allowed without prejudice to its restricted use under Annex III.
This update will be applied from October 1, 2021.
| Changes | Update | |
| Annex | Vi | |
| No. | 27' | |
| Substance Identification | Chemical name/INN | Titanium dioxide |
| INCI name | Titanium dioxide | |
| CAS no. | 13463-67-7/1317-70-0/1317-80-2 | |
| EC no. | 236-675-5/215-280-1/215-282-2 | |
| Restrictions | Product type, Body parts | |
| Max. concentration in ready for use preparation | 25%(4) | |
| Other | Titanium dioxide in powder form For the product types under letter | |
| Wording of conditions of use and warnings | ||
The use of Titanium Dioxide in Annex IV as a colorant should therefore be allowed without prejudice to its restricted use under Annex III.
This update will be applied from October 1, 2021.
| Changes | Update | |
| Annex | IV | |
| No. | 143' | |
| Substance Identification | Chemical name | Titanium dioxide |
| CI number | 236-675-5 | |
| CAS no. | ||
| EC no. | 236-675-5/215-280-1/215-282-2 | |
| Color | White | |
| Restrictions | Product type, Body parts | |
| Max. concentration in ready for use preparation | ||
| Other | Purity criteria as set out in Commission Directive 95/45/E (E 171) Titanium dioxide in powder form containing 1% or more of particles with aerodynamic diameter ≤ 10 μm, to be used in compliance with Annex III, No [321] | |
| Wording of conditions of use and warnings | ||
(C)
To align with the SCCS/1601/18 opinion published on June 20-21, 2019, entry of Salicylic acid in Annex III is therefore updated to include the following product types: body lotion, eye shadow, mascara, eyeliner, lipstick and roll-on deodorant with a maximum authorized concentration of use at 0.5%.
This update will be applied on the 20th day following its publication.
| Changes | Update | |
| Annex | III | |
| No. | 98' | |
| Substance Identification | Chemical name/INN | Benzoic acid, 2-hydroxy- |
| INCI name | Salicylic acid | |
| CAS no. | 69-72-7 | |
| EC no. | 200-712-3 | |
| Restrictions | Product type, Body parts | (a) Rinse-off hair products (b) Other products except body lotion, eye shadow, mascara, eyeliner, lipstick, roll-on deodorant (c) Body lotion, eye shadow, mascara, eyeliner, lipstick, roll-on deodorant |
| Max. concentration in ready for use preparation | (a)3.0% (b)2.0% (c)0.5% | |
| Other | (a), (b), (c) Not to be used in oral products | |
| Wording of conditions of use and warnings | (a), (b), (c) Not to be used for children under three years of age | |
(D)
The prohibition entry of Nickel bis(tetrafluoroborate) was introduced twice by error. Entry 1427 will therefore be removed.
This update will be applied on the 20th day following its publication.
| Changes | Keep | Delete | |
| Annex | III | ||
| Reference No. | 1401 | ||
| Substance Identification | Chemical name/INN | Nickel bis(tetrafluoroborate) | |
| CAS No. | 14708-14-6 | ||
| EC No. | 238-753-4 | ||
| Restrictions | Prohibited | ||
References
- Commission Regulation (EU) 2021/850
- Commission Delegated Regulation (EU) 2020/217
- SCCS/1617/20 opinion
- SCCS/1601/18 opinion
SGS technical experts have extensive knowledge and testing experience in materials and articles in contact with food. They work to ensure that your products meet the appropriate regulations for food contact materials, paving the way for compliance. From overall migration tests to expert advice on emerging regulations, compliance issues and documentation review, SGS is the partner to trust. In the end, it’s only trusted because it’s tested. Discover more on our website and read our brochure.
Next Step
With this regulation amendment, the cosmetic industry should particularly pay attention to the new requirement of Titanium dioxide in powder form containing 1 % or more of particles with aerodynamic diameter ≤ 10 μm. Cosmetic manufacturers are suggested to check the purity of Titanium Dioxide when raw material is purchased for production.
For inquiries, please contact:
Queenie Ho-yan TSE
Technical Service Manager
t: +852 2765 3672
© SGS Group Management SA - 2021 - All rights reserved - SGS is a registered trademark of SGS Group Management SA. This is a publication of SGS, except for 3rd parties’ contents submitted or licensed for use by SGS. SGS neither endorses nor disapproves said 3rd parties contents. This publication is intended to provide technical information and shall not be considered an exhaustive treatment of any subject treated. It is strictly educational and does not replace any legal requirements or applicable regulations. It is not intended to constitute consulting or professional advice. The information contained herein is provided “as is” and SGS does not warrant that it will be error-free or will meet any particular criteria of performance or quality. Do not quote or refer any information herein without SGS’s prior written consent.



