The European Medical Device Regulation (EU MDR) has redefined the landscape for medical device manufacturers, ensuring that products placed in the European Union (EU) market meet stringent safety and performance standards.
This regulation, which replaces the Medical Devices Directive (MDD) and the Active Implantable Medical Devices Directive (AIMDD), mandates comprehensive compliance for all medical devices. For manufacturers, understanding and adhering to EU MDR is not just a regulatory requirement but a critical component of market access and product safety.
Shoaib Ahmed, Medical Devices Lead Auditor at SGS Gulf Limited, UAE, provides expert insights into the MDR certification process, particularly for medical device manufacturers in the Middle East, offering a detailed guide to navigating these complex regulatory demands.
Why Compliance with EU MDR 2017/745 Is Essential
Every medical device manufacturer intending to sell products in the EU must comply with the EU MDR. This regulation introduces more stringent requirements than its predecessors, reflecting the latest advancements in technology and addressing the shortcomings of the previous directives. Compliance ensures that medical devices are safe, reliable, and effective, protecting patients and users across the EU.
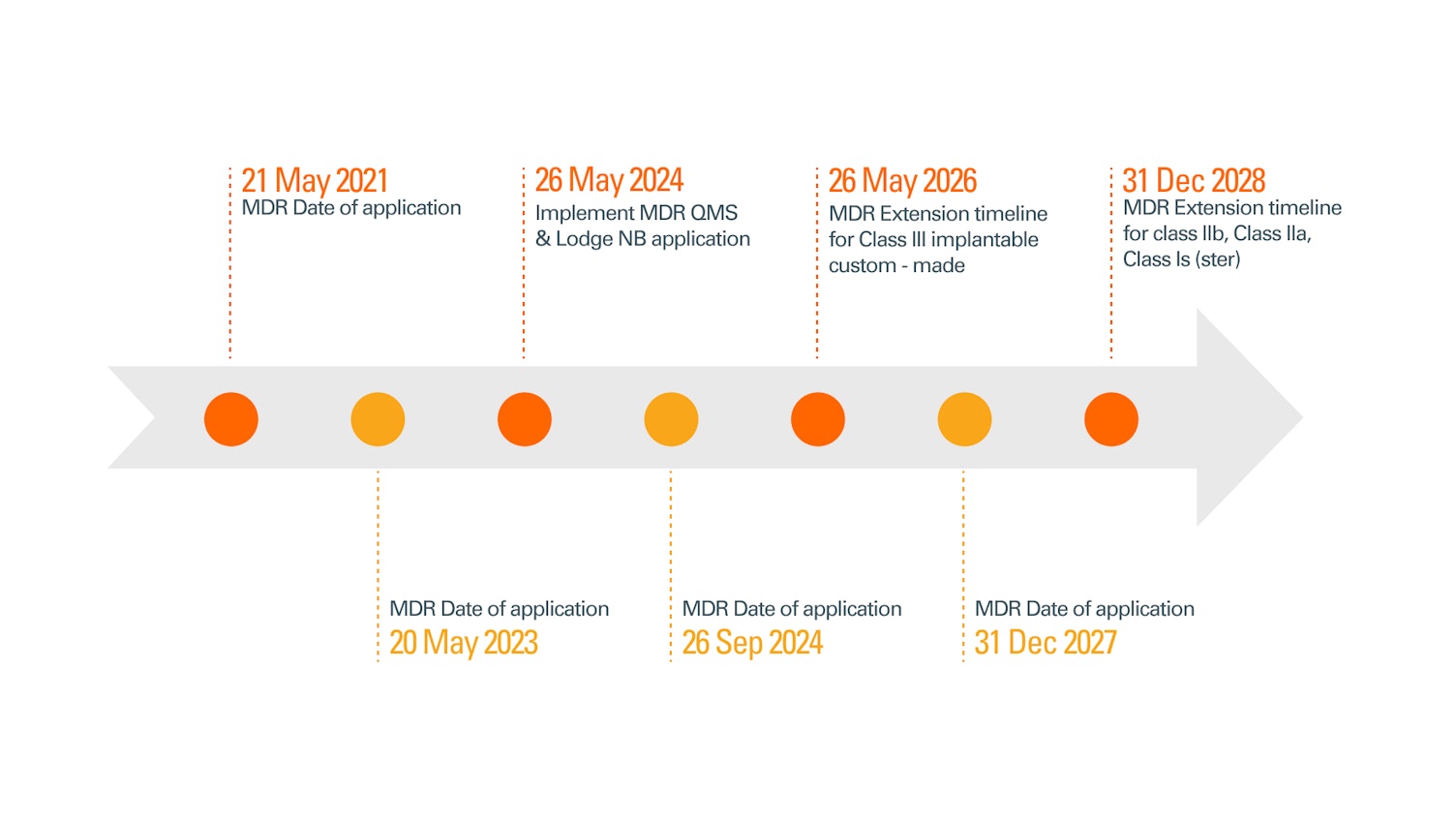
Key transition dates include:
31 December 2027: Compliance deadline for all Class III devices and Class IIb implantable devices (except for specific low-risk items such as sutures and dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips and connectors.).
31 December 2028: Compliance deadline for Class IIb devices not covered by point(a), as well as Class IIa devices and Class I devices placed on the market in sterile condition or having a measuring function devices.
New MDR Compliance timelines
All the manufacturers need to update their technical documentation from MDD/ AIMDD to MDR.
EU MDR remediation involves checking existing technical files against each MDR requirements. This involves gap analysis against new requirements, identification of new requirements /deliverables and remediation of MDD technical documentation.
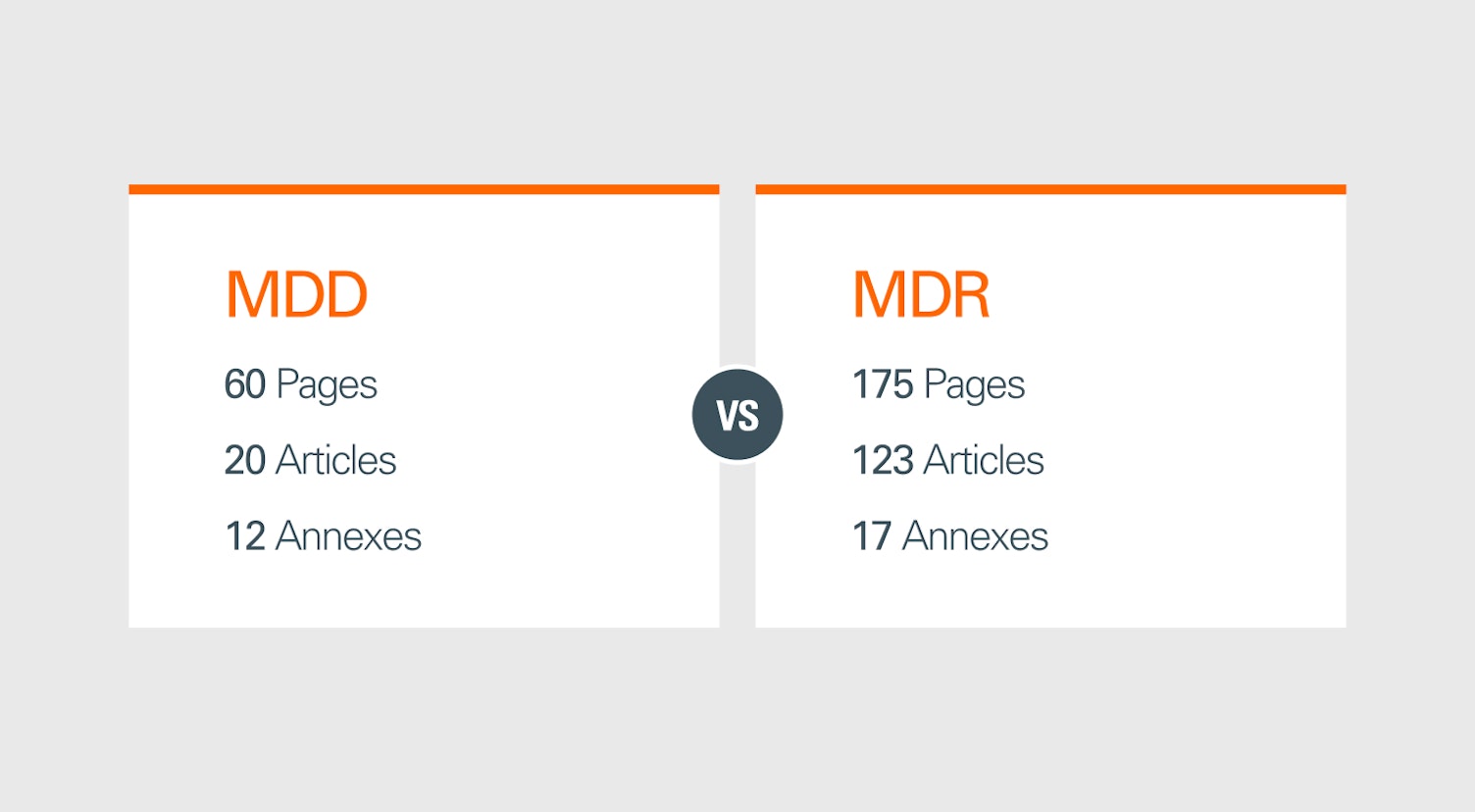
Key Changes from MDD to MDR
The shift from MDD to MDR is significant, impacting various aspects of medical device regulation. Below are some critical changes:
1. Legal Framework
Unlike the MDD, which was a directive allowing flexibility in transposition by EU member states, the MDR is a regulation with binding legal force across all member states from the set date of implementation.
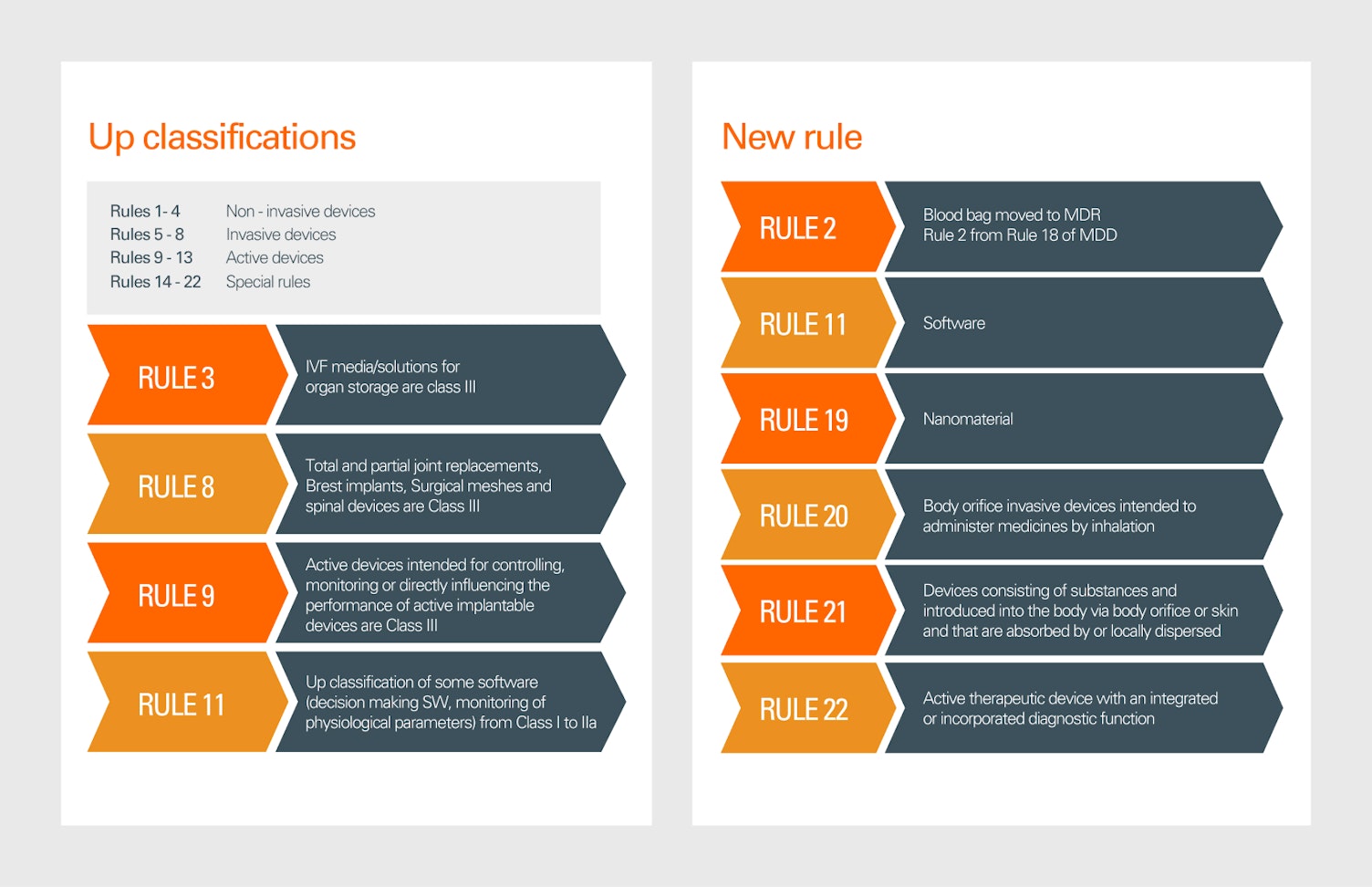
2. Device Classification
MDR introduces 22 classification rules, compared to 18 under MDD. This includes new definitions, the merging of existing rules, and the up classification of certain devices, making it imperative for manufacturers to reassess the classification of their products.
3. Traceability and Transparency
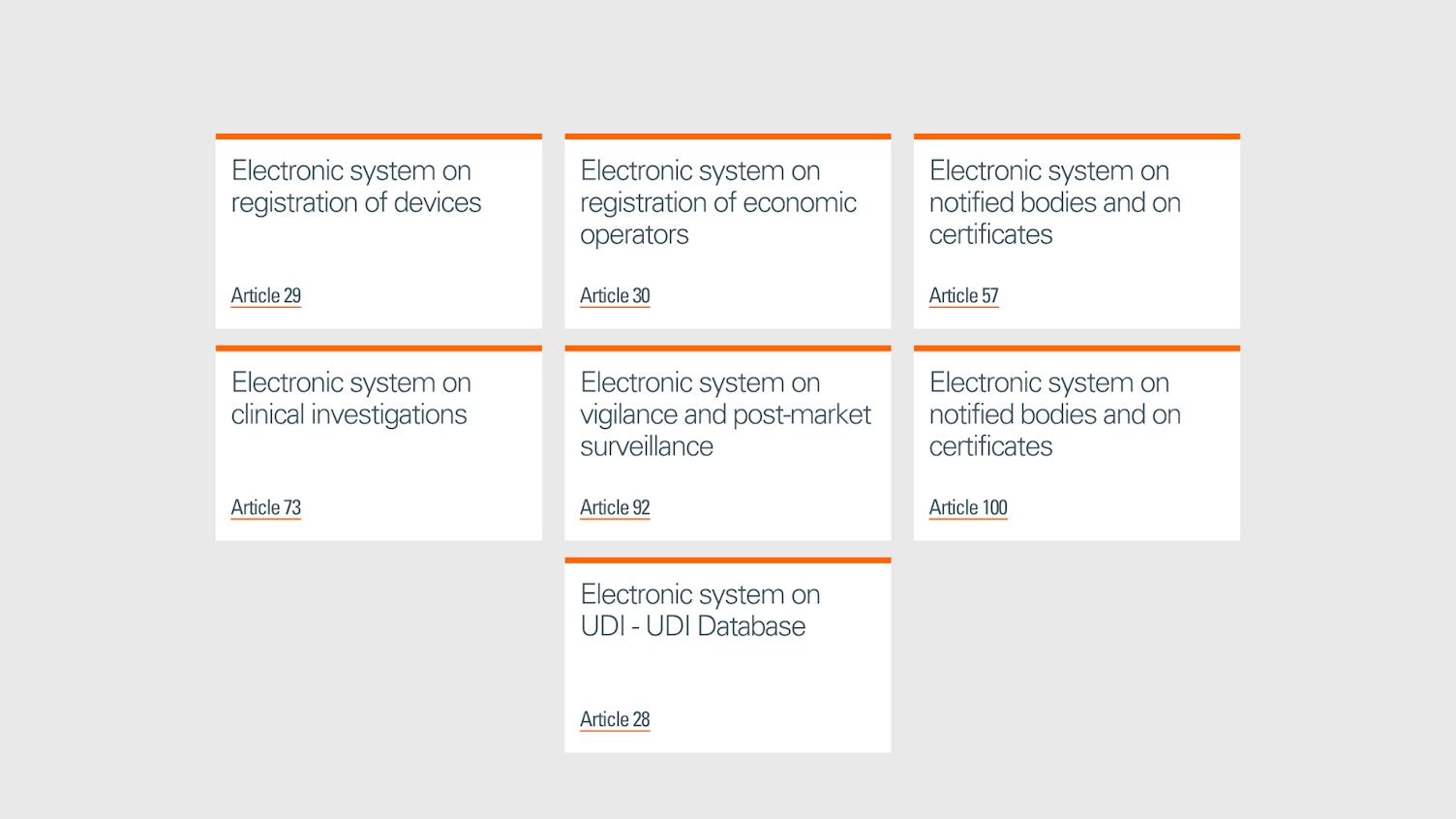
The MDR mandates the implementation of a Unique Device Identifier (UDI) for all medical devices, facilitating better traceability and monitoring throughout the device’s lifecycle. Additionally, the European Database on Medical Devices (EUDAMED) enhances transparency, linking various electronic systems for improved control and public access to information about medical devices.
4. Risk Management
MDR aligns closely with the harmonized standard EN-ISO 14971:2019, emphasizing the need to balance risk reduction efforts with the benefit-to-risk ratio. Manufacturers must update risk management documents to include new hazards, risk control measures, and considerations related to device lifetime.
5. Post-Market Surveillance (PMS)
Under MDR, PMS is more rigorous, requiring manufacturers to maintain detailed records and submit periodic safety update reports (PSURs) for higher-risk devices. This ensures ongoing compliance and safety monitoring even after the device has entered the market. The manufacturer must submit the trend reporting and there is an electronic system on Vigilance and PMS, this electronic system is linked with UDI and EUDAMED.
6. Labelling and Information
MDR imposes stricter requirements on labelling, ensuring clarity and completeness of information supplied with medical devices. This includes new symbols, updated instructions for use (IFU), and specific labelling requirements for devices with unique attributes, such as those containing medicinal substances or requiring sterile packaging.
The EU MDR Compliance Process
Transitioning to MDR compliance involves several steps:
- Gap Analysis: A thorough review of existing technical documentation against MDR requirements, identifying gaps and areas needing updates.
- Remediation: Updating technical files to address new MDR deliverables, such as risk management documents, clinical evaluation reports, and labelling requirements.
- Product Verification and Validation: Conducting rigorous testing and validation to meet MDR standards, including safety, biocompatibility, and Device Lifetime including shelf-life testing.
SGS: Your Partner for Comprehensive MDR Certification Services
SGS offers end-to-end support for your MDR certification journey. As a leading certification body with a strong presence in the Middle East, SGS provides expert guidance, training, and assessment services tailored to your needs.
- Local Expertise: With offices across Middle East and GCC countries, including UAE, Saudi Arabia, Qatar, Kuwait, Pakistan, Oman, Bahrain, SGS offers localized expertise, ensuring that your organization receives the best support in navigating MDR compliance.
- Training Courses: SGS’s dedicated training programs help your team understand the intricacies of MDR, ensuring they are well-prepared for the certification process.
- Certification Audits: SGS conducts thorough assessments and audits, ensuring your medical devices meet the highest standards of compliance.
Achieving MDR certification is a complex process that requires a strategic approach and expert guidance. By partnering with SGS, you ensure that your journey towards compliance is efficient, effective, and aligned with the latest regulatory standards. SGS’s comprehensive solutions not only help you achieve certification but also demonstrate your commitment to safety, quality, and market access in the EU.
For more information on how SGS can assist you with your MDR certification needs, contact us today.
About SGS
SGS is the world’s leading Testing, Inspection and Certification company. We operate a network of over 2,700 laboratories and business facilities across 119 countries, supported by a team of 99,250 dedicated professionals. With over 145 years of service excellence, we combine the precision and accuracy that define Swiss companies to help organizations achieve the highest standards of quality, safety and compliance.
Our brand promise – when you need to be sure – underscores our commitment to trust, integrity and sustainability, enabling businesses to thrive with confidence. We proudly deliver our expert services through the SGS name and trusted specialized brands, including Brightsight, Bluesign, Maine Pointe and Nutrasource.
SGS is publicly traded on the SIX Swiss Exchange under the ticker symbol SGSN (ISIN CH0002497458, Reuters SGSN.S, Bloomberg SGSN:SW).




