Why Antwerp has become a European hub for CHIM studies
Controlled Human Infection Model (CHIM) studies have moved from niche to strategic. Sponsors use CHIM because it delivers early answers with high decision value.
Antwerp has emerged as the place where CHIM studies move faster, with fewer dependencies and less regulatory uncertainty. It offers not only speed, but also predictability, infrastructure and experience. With several structural advantages converging, at the same time, Antwerp has become one of Europe’s most mature CHIM ecosystems. This is why:
Belgium treats biotech as infrastructure
Belgium’s biotech sector represents roughly 7% of national GDP. Biopharmaceuticals dominate export value. This means that early clinical research, including CHIM, receives sustained political and regulatory attention.
On the EMA’s own map of European clinical trial sites, Belgium appears almost saturated with expertise, despite its size. For sponsors, that density translates into experience: ethics committees, inspectors, regulators and investigators who are all familiar with early phase and high complexity studies.
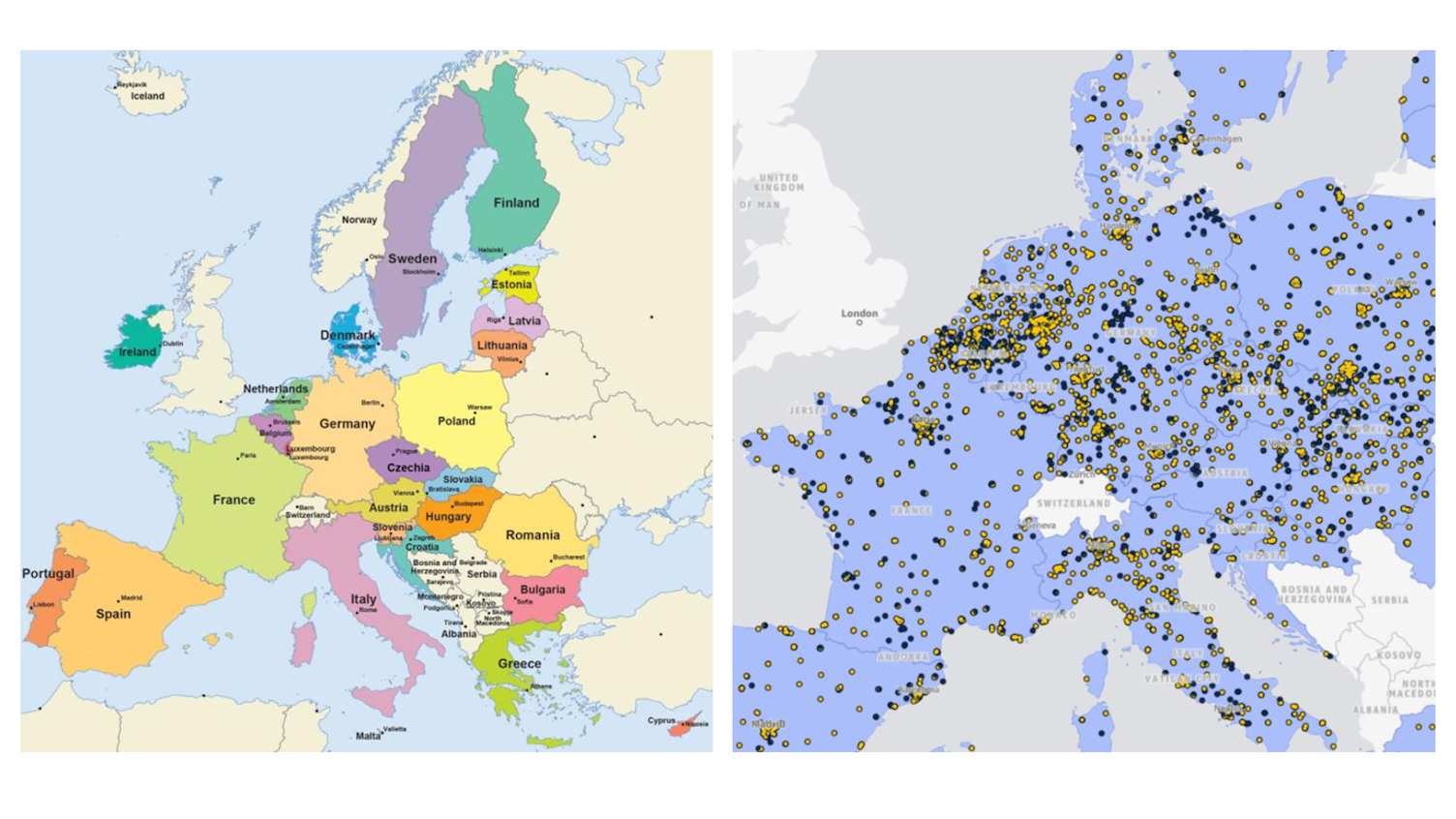
Source: euclinicaltrials.eu.
A concentrated CHIM ecosystem
One of Antwerp’s defining strengths is proximity.
Within a radius of 500 meters, two dedicated, complementary CHIM-capable units operate side by side. Our clinical pharmacology unit offers 46-bed BSL-2 capacity with integrated GMP manufacturing, QP release capacity and early phase execution. Vaccinopolis provides access to BSL-2 and BSL-3 capacity for higher-risk pathogens that cannot be handled in standard containment.
What does that mean for sponsors?
It means flexibility without fragmentation and collaboration without compromise. Studies can be designed around the scientific question rather than the limitations of a single facility. More complex challenge agents remain feasible. GMP manufacturing of the IMP, QP release and dispensing can happen close to the unit.

Regulatory timelines that reduce uncertainty
Belgium’s regulatory framework plays a central role in Antwerp’s attractiveness.
As explained in our webinar, Belgian law mandates accelerated approval timelines for mononational early-phase trials. Since January 2026, these timelines explicitly apply to Phase I, Phase I/II and Phase II mononational trials, including CHIM studies.
In practice, this caps approval timelines at 59 days from submission to authorization when RFIs occur and often less when dossiers are validated without questions. For monoclonal antibodies, Belgium limits extensions to 10 additional days, even where the EU regulation allows much more. The result is predictability.
Alongside accelerated timelines, the Belgian competent authority (FAMHP) continues to build regulatory expertise around advanced therapy medicinal products (ATMPs). For sponsors developing complex early phase programs, this regulatory depth supports clearer scientific dialogue and more predictable assessments, further reducing uncertainty compared with other European jurisdictions.
Proven experience across CHIM models
Antwerp’s position as a CHIM hub is not built on infrastructure and timelines alone. It rests on reliable challenge models with long-term reproducibility, giving pharma and biotech companies confidence when using CHIM data to support early efficacy and go or no-go decisions.
At our clinical pharmacology unit, sponsors can access viral challenge models, including influenza (H3N2), RSV (RSV-NICA), rhinovirus and malaria. The mature H3N2 influenza model has been deployed for more than a decade, showing consistent attack rates across seasons, age groups and genders. More recently, the novel RSV-A challenge model (RSV-NICA) has been validated. This challenge model achieved a 100% attack rate in its proof-of-concept cohort with a mild self-resolving pathology.
Beyond infectious agents, the unit also has long-standing experience with chemical challenge models, used to probe pharmacodynamic effects in a controlled and reproducible way.
A hub built for decision-making
Antwerp’s CHIM hub combines regulatory structure, physical proximity, operational maturity and long-term model experience in one location. For sponsors using CHIM as a strategic development tool to guide early go or no-go decisions based on early efficacy data, that combination makes all the difference.
About SGS
SGS is the world’s leading Testing, Inspection and Certification company. We operate a network of over 2,500 laboratories and business facilities across 115 countries, supported by a team of over 100,000 dedicated professionals. With more than 145 years of service excellence, we combine the precision and accuracy that define Swiss companies to help organizations achieve the highest standards of quality, compliance and sustainability.
Our brand promise – when you need to be sure – underscores our commitment to trust, integrity and reliability, enabling businesses to thrive with confidence. We proudly deliver our expert services through the SGS name and a portfolio of trusted specialized brands, including Applied Technical Services, Brightsight, Bluesign and Nutrasource.
SGS is publicly traded on the SIX Swiss Exchange under the ticker symbol SGSN (ISIN CH1256740924, Reuters SGSN.S, Bloomberg SGSN SW).