
EcoVadis Sustain 2026

Data Centre World London
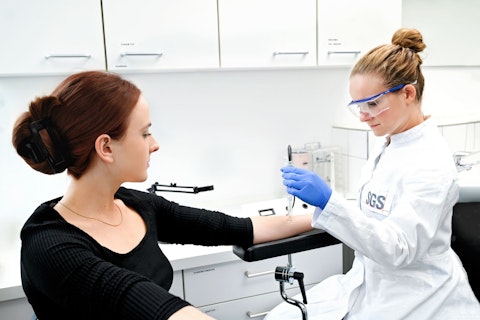
Electrical and EMC Safety in IVD Medical Devices
What should be considered in the requirements for an In Vitro medical device from the perspective of electrical safety and EMC? What requirements must be met before placing the product on the market? In this training, we will review the most essential requirements from the electrical safety and EMC standards EN 61010 and EN IEC 61326.

GFSI Conference 2026

Meet SGS at the European PFAS & Emerging Contaminants Summit 2026

SGS 2026 Annual General Meeting of Shareholders

SGS First Quarter 2026 Sales Update

Medical-Device Usability – IEC 62366-1 as part of the development process
Medical Software - ISO 13485 and IEC 62304 as Part of the Development Process

IEC 60601-1 as part of Medical Device Design and Testing
During the training we will open the content of the IEC 60601-1 series of standards in terms of technical requirements. We review IEC 60601-1 as part of its approvals for different markets. From IEC 60601-1 ed. 3.2, we provide you with an understanding of testing, electrical safety and risk management requirements for medical devices. All of which are key considerations in the design and testing of medical devices.